Detection of specific elements present in organic compounds by their atomic emission properties has been in use for many years and has now developed to the stage where a number of specific elements can be simultaneously detected.
The atomic emission detector (AED) is based on the intense emission properties of elements, particularly halogens, phosphorus, nitrogen, and sulphur, following excitation in a helium plasma. The plasma can be generated and maintained in a number of ways but the favored method is by microwave induction. Indeed in its early stages this detector was referred to as the microwave plasma detector.
Atomic emission detector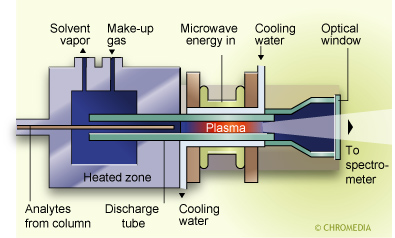
AED spectroscopic part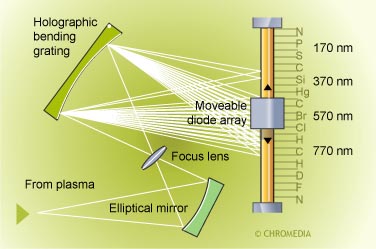
Detection limits for the AED depend on the particular element involved but are in the range of 0.1 - 75 pg/s with a linear range of about 104. For a gasoline sample, for instance, channel (a) can be set for carbon detection and (b) can be set to be specific for lead compounds. Other metal atoms can also be detected with high sensitivity and selectivity in presence of high concentrations of hydrocarbons.
A recently developed AED can monitor up to fifteen elements automatically by the use of a movable photodiode array detector.
 Did you ever try to explain separation to your employees or students? Well, try no more: Lee Polite did it for you in a way which is hard to beat. We will open up one example of his whiteboard class.
Did you ever try to explain separation to your employees or students? Well, try no more: Lee Polite did it for you in a way which is hard to beat. We will open up one example of his whiteboard class. 




